
Leading the Way in Revolutionary
Cannabinoid Medicines
Sanna Science is shaping the future of safe and effective breakthrough medicines by developing clinically-validated formulations of isolated cannabinoids.

Safe and Effective, Breakthrough
Treatments
Our vision is to leverage the untapped potential of cannabinoids in a systematic and clinically validated manner.
Our innovative formulations are built on specific isolated cannabinoids in precise ratios and are put through the full rigour of clinical trials to establish safety and effectiveness.
What We Do
Research and Development
We take a rigorous approach to working with isolated cannabinoids, designing formulations with specific, targeted effects based on the known properties of major and minor cannabinoids.

Clinical Validation
The safety and effectiveness of any therapeutic tool can only be established with the highest caliber of clinical trials. Taking our formulations to market as pharmaceutical IP means putting them through such validation.

Accessing Markets
The goal is always to take exciting new treatment options to market. Sanna Science has the experience and capacity to go from research to trials to production with offerings that have been developed, tested and shown to be safe and effective. We take the steps required to enter the regulatory channels that will give our products access to multiple markets as prescription-only medicines.

Sanna Sleep
Our active focus is on sleep, and the Sanna Sleep formulation represents the world’s first cannabinoid sleep capsule with successful clinical results.
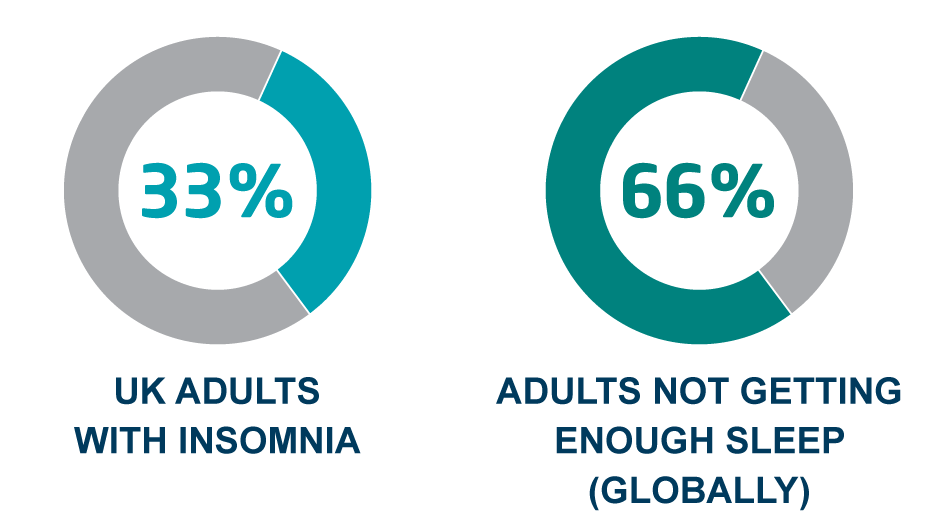
With roughly 2 in 3 of adults reporting that they do not get enough quality sleep, and 1/3 adults in the UK suffering with insomnia, the health, safety and productivity costs of sleep are enormous.
Despite the prevalence of these issues, there exists a significant treatment gap between problematic options like prescription benzodiazepines on one side and largely ineffective natural remedies with no clinical support on the other. Sanna Sleep bridges that gap.

The Right Partners
With its strong commitment to research-driven innovation in the pharmacological space and deep expertise in issues around sleep, King’s College London is a perfect fit to partner with Sanna Science.
Principal and contributing investigators from King’s College London will run the Phase 1b and PK clinical trials of the Sanna Sleep formulation.
The trials will be designed and executed by industry leader Lindus Health. Protocol design, patient recruitment, data collection and delivery will be handled by their team of experts.

Strategic advice and technical support from the Newmarket team will be essential for efficiently navigating market entry and regulatory processes, ultimately driving uptake of formulation post-clinical trial.